(Originally posted in ARSH 2020. Look at the tweet below added as an update in ARSH 2021 citing a MERE 26,000 vaxx deaths, sigh, and wax nostalgic. Latest super-lowball estimates are 17,000,000+ dead. That’s THREE of the “Worst Thing That Ever Happened Ever”. Are we even allowed to suggest the “The Worst Thing That Ever Happened Ever” at 6,000,000 might not be The Worst Thing To Ever Happen Ever? ESPECIALLY now? And it’s just getting warmed up. Nevermind the sterilizations that will go unrecognized and unrecorded.
I had the honor of organizing the Sacrament of Extreme Unction for an elderly friend going into the hospital today for cancer surgery tomorrow. The elderly, especially, were so vulnerable to the relentless satanic propaganda about the mRNA poison via TeeVee. Is his cancer “turbo cancer”? Probably. In your charity, could you say an Ave for my friend Joe? A wonderful oldtimer. Was Airborne in the late-60s. He was tricked. Pure and simple. He wants to continue to live and work, and see his grandchildren grow up. I want that too, but more importantly I want him to go to heaven. -AB ’24)
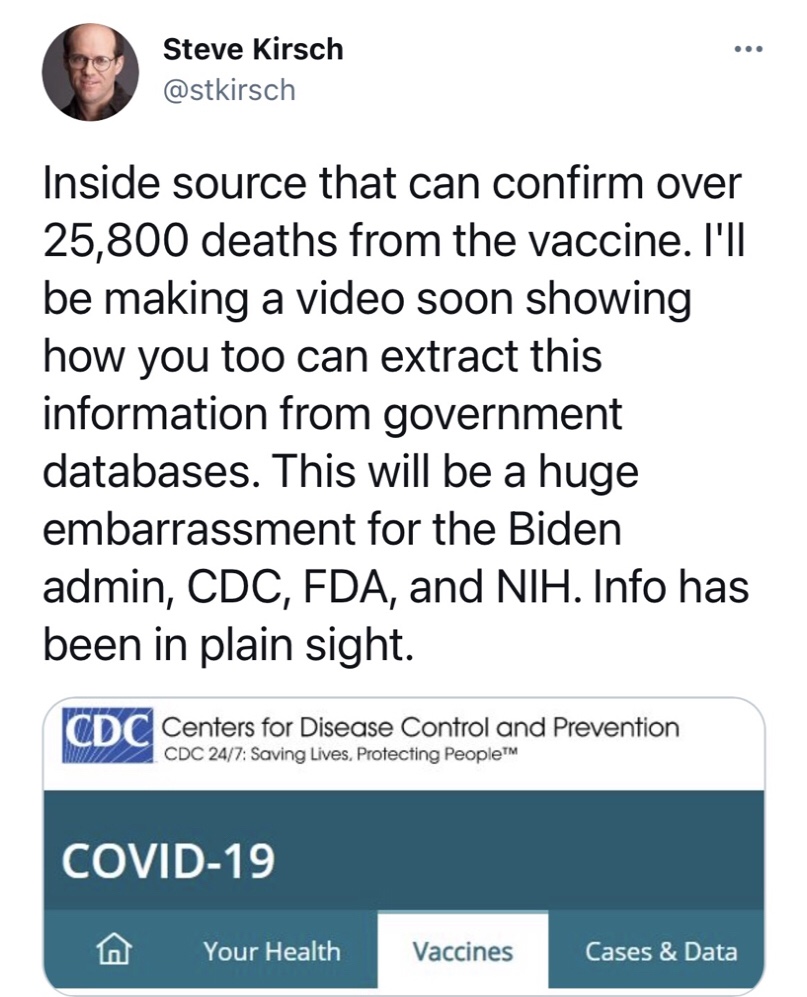
Folks, every bit of this has been METICULOUSLY pre-planned and anticipated down to the smallest details. Read this excerpt from the master planning document of the CoronaScam Crime Against Humanity in light of the rapidly growing numbers of the dead, maimed and injured by the DeathJab and realize that it is ALL PART OF THE PLAN.
Folks, everything here below was published in ARSH 2017 as a FICTIONAL WAR GAMING SCENARIO which now reads as an obvious and undeniable SCRIPT for a meticulously conceived and pre-planned crime against humanity. There is NO POSSIBLE WAY that what you read below is a coincidence. None.
Understand that mass injuries and death from a “vaccine” are 100% anticipated, down to the craven plans to silence the victims and mollify the masses through manipulative media coverage, sentimental gestures and financial payoffs, but that the injections MUST CONTINUE.
I have included, in full, the final three chapters of the document, which begins on document page 59, PDF page 68.
Again, remember as you read this that it was published in ARSH 2017 as a “hypothetical” war gaming scenario.
VACCINE INJURY
CHAPTER SEVENTEEN
In contrast to Alyssa Karpowitz’s story, not all changes in opinion were in favor of public health messaging. As time passed and more people across the United States were vaccinated, claims of adverse side effects began to emerge. Several parents claimed that their children were experiencing neurological symptoms similar to those seen among livestock exposed to the GMI vaccine. By May 2027, parental anxiety around this claim had intensified to the point of lawsuits. That month, a group of parents whose children developed mental retardation as a result of encephalitis in the wake of Corovax vaccination sued the federal government, demanding removal of the liability shield protecting the pharmaceutical companies responsible for developing and manufacturing Corovax.
The growing plaintiff cohort quickly withdrew their suit upon learning that the National Vaccine Injury Compensation Trust Fund (NVICTF) and an emergency appropriation of funds authorized by Congress under the PREP Act existed to provide financial reimbursement to those who were adversely affected by the Corovax vaccine in order to cover healthcare costs and other related expenses.2,3 Given the positive reaction to the federal government’s response and the fact that the majority of US citizens willing to be vaccinated had already been immunized, the negative publicity surrounding adverse reactions had little effect on nationwide vaccination rates. The focus on adverse side effects, however, resulted in a considerable increase in the number of compensation claims filed, and many grew concerned about the long-term effects that Corovax could have on their health. This concern was particularly high among some African American parents who continued to question the government’s motives regarding the Corovax vaccination campaign.
While the FDA, CDC, and other agencies were busy researching possible connections between Corovax and the reported neurological side effects, their efforts were continually undermined by epidemiological analyses produced by various non-governmental individuals and groups. A popular science blogger EpiGirl, for example, began posting interactive maps of the incidence of Corovax side effects in April 2027. To create the maps, EpiGirl collected anecdotes of adverse Corovax side effects using Facebook, Twitter and YouTube and combined them with data downloaded from the HHS Vaccine Adverse Event Reporting System (VAERS), a national vaccine safety surveillance program maintained by the CDC and FDA. EpiGirl also encouraged those among her subscribers who were Apple product users to share health data with her via Apple’s ResearchKit and HealthKit applications. EpiGirl’s maps were consequently shared widely in social media circles and even included in local and national news reports.
The federal government became concerned about the validity of EpiGirl’s anecdotal data and the widespread sharing of patient information via the internet. EpiGirl’s data showed a significantly higher incidence rate of nearly every reported side effect; however, federal officials believed that this was largely due to duplicate entries resulting from compiling data from multiple sources. Additionally, EpiGirl’s data did not seek to address the cause of the reported side effects, only the incidence rate. Publication of similar results from organizations such as Patients-Like-Me, a group closely associated with the natural medicine movement, further legitimized these independent reports. The government attempted to respond to these claims through formal press releases, but these were neither as visually appealing nor as interactive as EpiGirl’s maps and were, therefore, largely ignored.
While the federal government appeared to have appropriately addressed concerns around the acute side effects of Corovax, the long-term, chronic effects of the vaccine were still largely unknown. Nearing the end of 2027, reports of new neurological symptoms began to emerge. After showing no adverse side effects for nearly a year, several vaccine recipients slowly began to experience symptoms such as blurry vision, headaches, and numbness in their extremities. Due to the small number of these cases, the significance of their association with Corovax was never determined. As of this writing in 2030, longitudinal studies initiated by the NIH at the beginning of the vaccination program have not reached the next round of data collection, so formal analysis on these symptoms has not yet been conducted. Furthermore, these cases arose from the initial cohort of vaccine recipients—those in high- risk populations, including those with other underlying health conditions—making it increasingly difficult to determine the extent to which these symptoms are associated with vaccination.
As these cases emerged, patients began filing for compensation under the PREP Act. Due to lingering uncertainties over possible links between vaccination and reported neurological symptoms, their compensation requests were placed on indefinite hold, pending further data analysis. This cohort, many of whom adamantly supported the Corovax vaccine initially, quickly took to social media to publicize their issues.
Despite relatively few reports of neurological symptoms, the social media response was immense. After experiencing initial success with PREP Act compensation policies and working diligently to ensure transparency throughout the claim request and evaluation process, HHS was caught off guard by the new round of negative publicity. They were pressured by the public and media to award compensation to those claiming long-term effects from Corovax despite having no data to support these claims. Displaying a fundamental misunderstanding of scientific research, many demanded proof that the vaccines did not cause long-term effects. HHS Secretary Nagel firmly and vocally supported the decision to postpone evaluation of all claims of long-term side effects and invited an independent Congressional investigation to ensure that the PREP Act was being properly implemented.
In addition to demands for immediate compensation, Congress faced public pressure to increase the PREP Act emergency appropriation. While the initial allocation of funds was sufficient to provide compensation for acute side effects, the prospect of long-term effects and potentially permanent disability gave rise to concerns that additional resources would be necessary in the near future.
COMMUNICATION DILEMMA
Communicating With the Public About Trustworthy Sources of Data and
Options for Legal Recourse in a Climate of Mistrust
FOOD FOR THOUGHT
1) How might advance development and testing of recovery messages that specifically address the topics of adverse side effects and the NVICTF help improve health authorities’ ability to respond to public distress about medical issues emerging after a MCM campaign? What are some messages that would warrant such testing?
2) Despite the uncertain science about the link between Coravax and the reported neurological symptoms, why should health officials still communicate with compassion and genuine sympathy toward those in the vaccinated population who experience medical issues subsequent to being vaccinated?
3) Given growing interest in open data systems and the application of “crowd sourcing” to solve complex problems, how might public health officials take greater advantage of two-way communication with an interested public in the aftermath of the SPARS outbreak? For instance, how might input and analysis from members of the public help improve adverse event monitoring or assess the strengths and weaknesses of a specific MCM campaign?
ACKNOWLEDGING LOSS
CHAPTER EIGHTEEN
At the request of HHS Secretary Nagel, ASPR convened a series of meetings among senior leadership of the federal health agencies to address policy and program changes being implemented as a result of a departmental review of the response to the SPARS pandemic. Among the issues considered were the implications of growing negative public opinion regarding Corovax and the government’s perceived indifference to victims of the public health response to SPARS. One senior health official argued that time and a robust medical monitoring program for vaccine recipients—the components of which were already in place—should be sufficient to determine whether public concern about long-term effects was, in fact, warranted: “We have to wait for the data. People need to understand that fact.”
One prominent attendee at these meetings was Dr. Ann Flynn, the director of the Substance Abuse and Mental Health Services Administration (SAMHSA). Staff from the administration’s Disaster Technical Assistance Center had recently briefed Dr. Flynn on usage data for the SAMHSA Disaster Distress Helpline over the past year, and summary reports indicated that a significant number of helpline users said that their principal worry was associated with the SPARS pandemic and, more recently, uncertainty about potential long-term effects of Corovax. Considering this new knowledge, Dr. Flynn countered the earlier claim that the public simply needed to wait until the science was clear: “Communities around the country went through what some felt was a harrowing public health emergency, only later to confront the possibility, however slim, that the medicine we promised would help them may in fact be hurting them.”
The senior leaders in attendance concluded, after much prompting by Dr. Flynn, that no top political or public health figurehead had publicly recognized the collective sense of vulnerability that the pandemic had elicited or the strength that the public exhibited under threat of grave danger. Moreover, no national leader had publicly acknowledged the public’s broad willingness to accept a prescribed countermeasure that promised to end the pandemic, but whose long-term consequences were not fully understood at the time.
Following the meeting, ASPR recommended to HHS Secretary Nagel that SAMHSA collaborate with stakeholders and devise behavioral health guidance for the states, tribes, and territories on how to strengthen the public’s coping skills, provide support for grieving individuals, encourage a forward direction, and meet other SPARS recovery needs. It was further recommended that Secretary Nagel consult with President Archer about the possibility of acknowledging the emotional toll of SPARS during a future public appearance. The primary message would be one of gratitude to the American people for remaining strong during the pandemic. Another key message would convey appreciation for adhering to public health recommendations, including vaccination, to hasten the end of the pandemic in the face of considerable uncertainty.
President Archer agreed to address the country’s resolve and recovery in the face of SPARS. Top risk communication advisors from the CDC, FDA, NIH, and SAMHSA conferred as a group about how best to frame the President’s remarks. The group vigorously debated whether it was appropriate for the President to acknowledge the sacrifice that vaccine recipients had made on behalf of their communities or to console them in their grief over that sacrifice.
COMMUNICATION DILEMMA
Bringing a Sense of Resolution to a Period of Crisis While Striking a Balance Between the Need to Affirm Collective Grief and Loss and the Need to Move Forward
FOOD FOR THOUGHT
1) Given the uncertain long-term safety profile of the Corovax vaccine, why are both science and sympathy necessary when communicating about a possible correlation between vaccination and adverse events?
2) What general communication principles does the advice of Dr. Ann Flynn suggest with respect to the recovery phase of a public health emergency involving MCMs? What might pre-event planning for recovery-phase communication look like based on her guidance?
SPARS AFTERMATH
CHAPTER NINETEEN
Today, nearly five years since the St. Paul Acute Respiratory Syndrome coronavirus made its global debut, there remain human cases in 14 countries across Europe, Africa, and Asia. The pandemic officially ended in August 2028, but the virus persists in domesticated animal reservoirs. WHO experts hypothesize that small, isolated outbreaks of SPARS were occurring long before the disease emerged on a global scale in 2025, and they anticipate that future outbreaks will continue to emerge unless countries maintain widespread vaccination coverage.
As the pandemic tapered off, several influential politicians and agency representatives came under fire for sensationalizing the severity of the event for perceived political gain. As with many public health interventions, successful efforts to reduce the impact of the pandemic created the illusion that the event was not nearly as serious as experts suggested it would be. President Archer’s detractors in the Republican Party seized the opportunity to publicly disparage the President and his administration’s response to the pandemic, urging voters to elect “a strong leader with the best interests of the American people at heart.” A widespread social media movement led primarily by outspoken parents of affected children, coupled with widespread distrust of “big pharma,” supported the narrative that the development of SPARS MCMs was unnecessary and driven by a few profit-seeking individuals. Conspiracy theories also proliferated across social media, suggesting that the virus had been purposely created and introduced to the population by drug companies or that it had escaped from a government lab secretly testing bioweapons.
After-action reports, government hearings, and agency reviews following the pandemic were too numerous to count. Emergency funding appropriated by Congress to fight the disease became available partway through the course of the pandemic, but federal, state, and local public health agencies struggled to manage the procedural requirements to spend it. As a result, significant amounts of emergency funds remained unused as the pandemic wound down. As the investigations grew in intensity, several high-ranking officials at the CDC and FDA were forced to step down and withdraw from government in order to “spend more time with their families.” Exhausted employees of these agencies, many of whom worked long hours six or seven days a week throughout the pandemic, simply wanted to put the whole response behind them. Little desire remained on the part of decision-makers or those who served in the trenches during the response to rehash the events of the past several years.
The very real possibility of a future SPARS pandemic necessitates continued commitment to vaccination programs as well as accurate, culturally appropriate, and timely communication from public health agencies across the planet. While the communication experiences of the SPARS pandemic of 2025-2028 offer some examples for how this communication can and should occur, they also identify practices that should be avoided, or at least modified, for responses to future public health emergencies.